The healthcare industry is undergoing a digital transformation, and regulatory affairs—traditionally one of the most complex and resource-intensive functions—are now at the center of this shift. As global regulations become more stringent and drug development timelines tighten, artificial intelligence (AI) is emerging as a powerful enabler in healthcare regulatory affairs. By automating compliance processes, enhancing data accuracy, and improving submission efficiency, AI is redefining how pharmaceutical, biotechnology, and medical device companies interact with regulatory bodies.
Market Overview and Growth Outlook
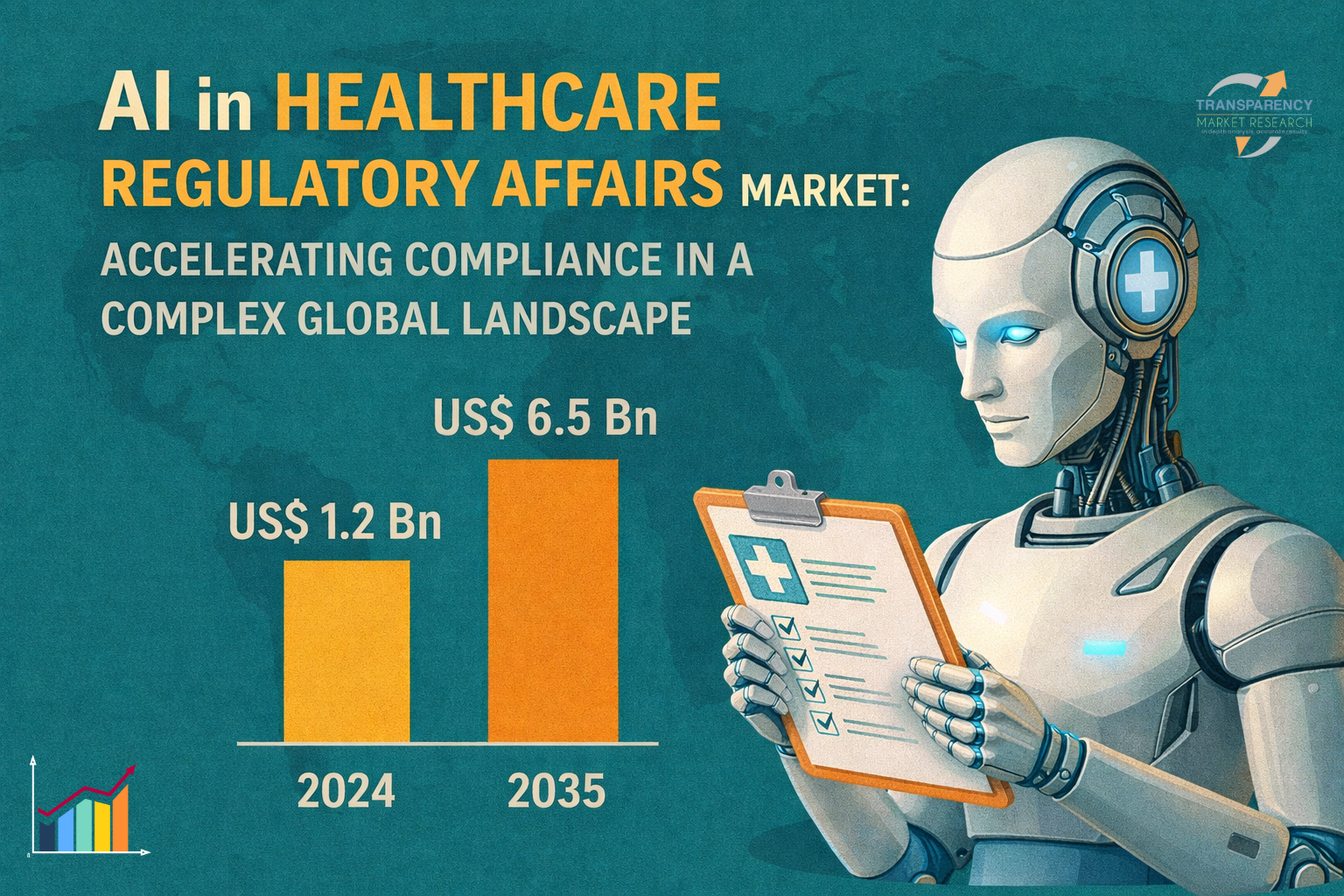
The global AI in healthcare regulatory affairs market was valued at US$ 1.2 billion in 2024 and is projected to reach US$ 6.5 billion by 2035, expanding at a robust CAGR of 16.7% from 2025 to 2035. This rapid growth reflects the increasing reliance on AI-driven platforms to manage regulatory complexity, reduce approval delays, and minimize compliance risks across global markets.
Regulatory authorities worldwide continue to introduce evolving frameworks, data requirements, and reporting standards. AI-powered solutions help organizations adapt quickly by analyzing large regulatory datasets and identifying changes that may impact submissions or post-market obligations.
Rising Complexity of Global Regulations
One of the primary drivers of AI adoption in healthcare regulatory affairs is the growing complexity of international regulatory environments. Companies operating across multiple regions must comply with varying standards set by agencies such as the FDA, EMA, MHRA, and other national authorities.
AI tools enable real-time tracking of regulatory updates, policy changes, and guidance documents across jurisdictions. Natural language processing (NLP) algorithms can analyze regulatory texts, extract key requirements, and alert regulatory teams to potential compliance gaps. This capability significantly reduces the risk of non-compliance while improving operational efficiency.
Improving Speed and Accuracy in Regulatory Submissions
Speed to market is critical in healthcare, particularly for innovative therapies and life-saving medical technologies. Traditional regulatory workflows often involve manual document preparation, review, and validation—processes that are time-consuming and prone to error.
AI-driven platforms streamline regulatory submissions by automating data aggregation, document formatting, and validation checks. Machine learning models can flag inconsistencies, missing information, or formatting issues before submission, reducing the likelihood of regulatory queries and resubmissions. This leads to faster approval timelines and improved collaboration between regulatory teams and product development units.
North America Leads Global Adoption
North America dominated the AI in healthcare regulatory affairs market in 2024, holding a 41.6% revenue share. The region’s leadership is driven by early adoption of digital health technologies, a strong presence of pharmaceutical and biotech companies, and a well-defined regulatory environment.
Regulatory agencies in North America increasingly support the use of advanced analytics and digital tools, further encouraging industry-wide adoption. Meanwhile, Europe and Asia Pacific are emerging as high-growth regions as companies modernize regulatory operations and expand into global markets.
AI Applications Across the Regulatory Lifecycle
AI technologies are transforming multiple stages of the regulatory lifecycle. In regulatory intelligence, AI systems continuously monitor global regulatory updates and assess their relevance to specific products. During submission preparation, AI automates document compilation, cross-referencing, and quality checks.
Post-approval, AI supports pharmacovigilance and compliance monitoring by analyzing real-world data, adverse event reports, and audit findings. Predictive analytics help organizations identify potential regulatory risks early, allowing proactive mitigation strategies.
Competitive Landscape and Leading Players
The AI in healthcare regulatory affairs market is moderately fragmented, with both established technology providers and specialized regulatory intelligence firms competing on innovation, data accuracy, and platform scalability.
Leading players operating in the global market include Clarivate, IQVIA Inc., Wipro, Freyr, Innoplexus, Zenovel, Indegene, RegDesk, Inc., CELEGENCE, Rimsys, DDi, DXC Technology Company, and Ketryx Corporation. These companies focus on enhancing AI capabilities such as predictive risk modeling, intelligent document management, and real-time regulatory intelligence.
Strategic partnerships between AI vendors and healthcare organizations are becoming increasingly common, enabling customized solutions tailored to specific regulatory requirements and product portfolios.
Challenges and Adoption Barriers
Despite strong growth potential, challenges remain. Data privacy concerns, integration with legacy systems, and the need for regulatory validation of AI tools can slow adoption. Additionally, regulatory teams must develop digital competencies to fully leverage AI-driven platforms.
However, as AI models become more transparent and regulatory agencies provide clearer guidance on digital tools, adoption barriers are expected to decline.
Future Outlook
The future of AI in healthcare regulatory affairs is defined by automation, intelligence, and predictive capabilities. As regulatory demands intensify and global product launches increase, AI will play an essential role in ensuring compliance while accelerating innovation.
Organizations that invest early in AI-powered regulatory platforms will gain a competitive advantage by reducing time-to-market, minimizing compliance risks, and improving regulatory decision-making. Over the next decade, AI is expected to evolve from a support tool into a strategic pillar of healthcare regulatory operations.